Eye – or in this case ear – opening podcast tackling the pain points with most process challenge devices. Bottomline of the podcast: IS IT REALLY CLEAN AND HOW EFFECTIVE IS YOUR CURRENT PROCESS CHALLENGE DEVICE?
Considering there are many different types of washers, we need a test device that analyses all the different approaches. (e.g. does the washer use spray arms or are the instruments physically connected to the washer allowing the water to flush? Are the surgical instruments shadowing each other? And are they in 3D or flat? Etc.)
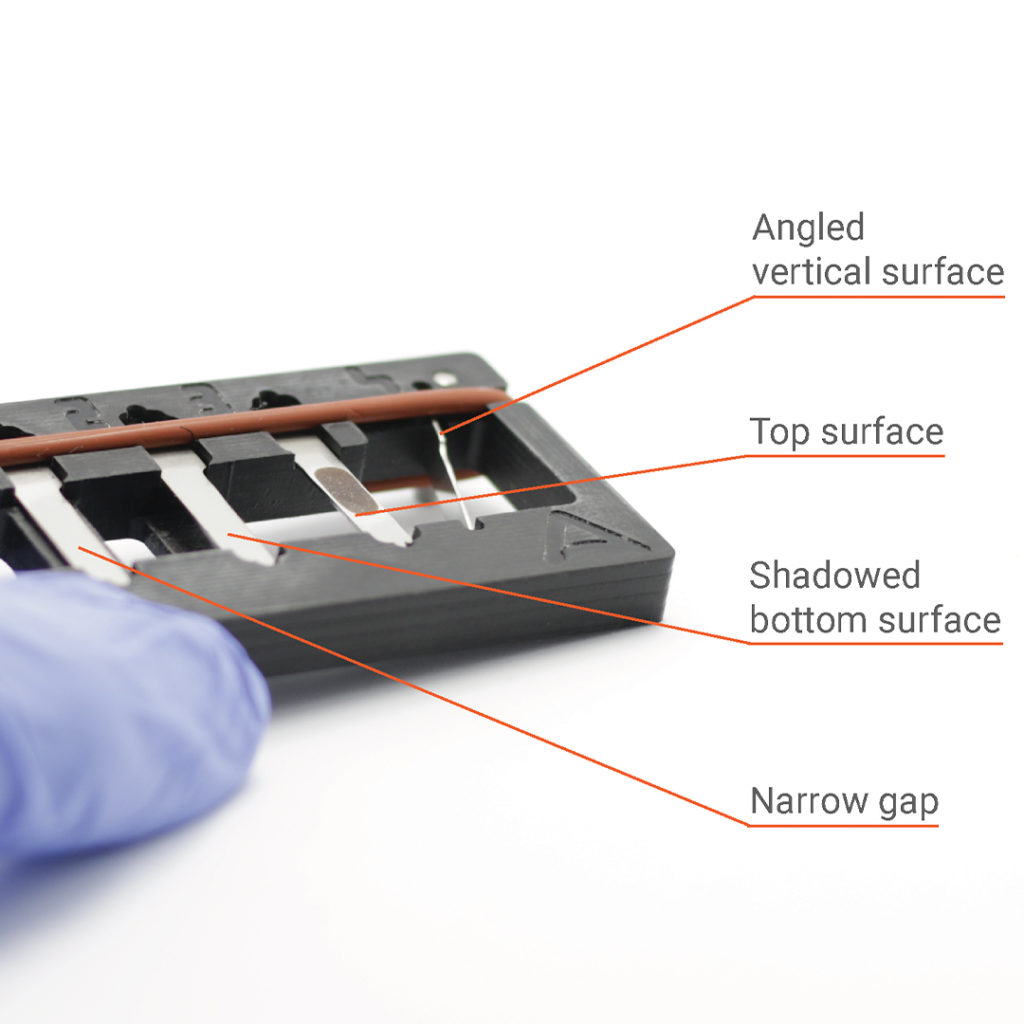
After years of research, Pawel De Sternberg Stojalowski and his Aseptium-team developed their innovative VeriTest. This unique cleaning verification kit is especially designed to analyze the effectiveness of multiple cleaning challenges at the same time: shadowed bottom surface, narrow gap, angled vertical surface and top surface.
Interested? Since UltraZonic is the distributor of Aseptium, we are happy to answer all your questions via info@ultrazonic.com.
For the busy bees among us: we’ve summed up some remarkable quotes:
“We need to challenge the cleaning process: Is it the chemical? Is it the way you load it? Is it perhaps the combination of the cycle? Use the pcd’s and try to make them fail so you can understand the process much better.”
“What makes a good challenge process device and what features would we like to have that resemble the modern complex instruments. First, we need to look if the machine uses sprinklers to clean or are we connecting the instruments physically to the washer and allowing the water to flush. Those are 2 completely different processes, so they must be evaluated separately. = This is the focus point!”
“Very often we have washers with different types of chemistry & we would like our test soil to be as realistic as possible. We are operating on tissues, and it are the tissues with all the different types of elements that get us contamination on our instruments. These are different kind of materials that give us contamination on our instruments. Think about the soft tissues of the operation table. This leaves residues on instruments that are NOT blood. And this is exactly why we must ensure that the test soil should really represent that type of challenge.”
“A good pcd looks at the narrow gaps and shadowing (one part of the instrument sits on the other part of the instrument and blocks the access of the spray). And are our instruments 3D or flat? Because then we need to clean from the sides, underneath and above. If you are not checking shadowing is when you maximize the lading because your bcd is always telling you it’s fine. But if you give it a closer look, you’ll notice that this is not always the case.”
“The true value of a pcd is not when they pass but when they fail, and HOW they fail. This is a cost, so we need to maximize the feedback. This is only possible when we know by are pcd fails. Challenge your process: Is it the chemical? Is it the way you load it? Is it perhaps the combination of the cycle? Use the pcd’s and try to make them fail so you can understand the process much better.”
“It’s about ensuring that we receive quality feedback from your washing and cleaning process. This is pretty much the last check you do before releasing your instruments to the next step. Only properly cleaned instruments can be safely sterilized.”